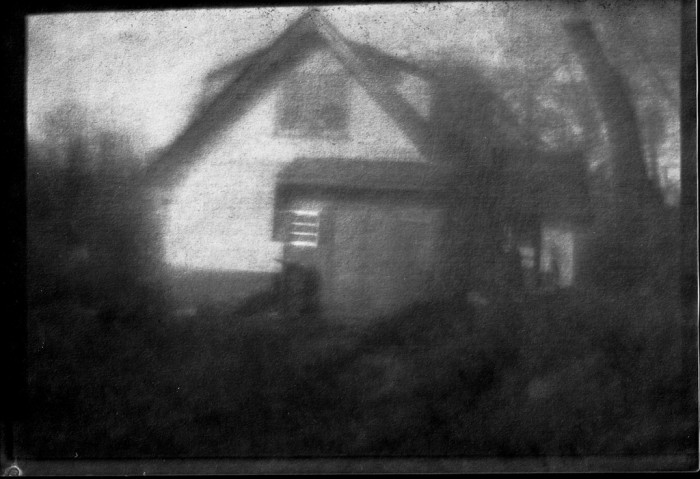Cyanotypes are one of the easiest alt process prints to make. Bought as a kit from just about anywhere, you get two bottles: part “A”
I enjoy printing cyanotypes: simple chemistry, simple reactions, nice deep blues, non-toxic, and the moment you drop it in water is dramatic. It’s very straightforward
This is the project that took up most of my 2012. And it’s what I wanted to post about repeatedly during the year, but my
Gum over cyanotype on a half sheet of Stonehenge Rising warm white paper.
This print is a direct result of a morning of failures last week. My cyan pigment wouldn’t stay on the paper – a combination of two different problems related to two variables I changed simultaneously. After three poor prints I decided I would forego the cyan for the morning and use cyanotype for the base layer to get me started.
I miscalculated the amount of cyanotype solution I needed (resulting in the …[more]
This one takes some explaining; it’s some of what’s been eating up my time for the past few weeks.
This shot started life on HP5+ developed in Xtol. I digitally inverted and enlarged the shot and printed it on Pictorico Ultra Premium OHP before stuffing it in to my vacuum frame (yes, that shot shows me mis-printing a positive I printed first – oops).
Traditionally, Cyanotype has a “part A” (green ferric ammonium citrate) and a “part B” ( …[more]
This is a “New” Cyanotype, from Mike Ware’s modernized Cyanotype formulation. The image may look familiar to long-time viewers; it’s a rework of a previous image, direct-printed on to an 8.5×11 sheet of cyanotype-sensitized paper. Exposure time was around 5 minutes in direct (winter, low-sky) sunlight.
Next week, I’ll be teaching a room of 4 and 5 year olds a little bit about film photography. I’ll be giving them disposable cameras to shoot with, and figured I’d build a Camera Obscura to show them what’s going on inside the camera (and why they can’t see the picture as soon as they snap the shutter). I had been thinking about how I could load it up with some sort of film that didn’t require a toxic developer, when a co-worker reminded me about “Sun Print” paper. This stuff is some form of c …[more]